Soil and Germination:
This post is some of my research around soil water, and soil structure. I don't guarantee accuracy especially with some terminology I am probably using wrong.
With greenhouse germination there are several routes:
- Geoponics
- Growing plants in substrate (Peat, Coco noir, pumice, clay)
- Hydroponics
- Growing plants in water
- Flooding and empty cycles
- Trickle flow
- Aeroponics
- Growing plants suspended in air, spraying with water
When thinking about the needs of your plants you should know a bit of soil science.
- Hysteresis - how a soil wets and drys
- Stratification, do I put gravel at the bottom of my pot
- Nutrients in a soil and soil pH
- Organic, do I even need it?
Soil science is not my forte, but I've tried to research this thoroughly. If you see mistakes, especially in terminology feel free to comment or email me with suggestions (no time for sass!)
1. Hysteresis
You have a cup of water with a pot in it. The water level in the cup we'll call zero cm of water (in black below, we'll measure the level of water in the soil/substrate from this level.
On the below left a course soil, mostly gravel (>2 mm), on the right a finer soil with sand ( 0.05 - 2 mm). Water is effectively pulled through a soil by it's adhesion to substrate and it's held back by cohesion to itself.
If you drop a bead of water on a surface, it can stay as a bead as long as the water surface tension, i.e. cohesion, is greater than the water's adhesion to substrate. If you drop a bead of water on a wood table it stays a little dew drop because of cohesion. If you drop a bead of water on cotton fabric, the water adheres to the fine network of fibres and spreads out.
In the illustration below we see capilliary action as a balance between adhesion and cohesion. Gravel has bigger pores, bigger spaces between the gravel particles which allows a balance of cohesion to adhesion such that there is only a small capillary action drawing water up. In sand there is so much negatively charged surface area the water clings to more particles through adhesion and overcomes its surface tension, i.e. cohesion, getting drawn up into the pot higher.
What does this mean in developing a soil to grow lithops? Well in my case I thought it best to test out different grades of porous and non porous substrate to understand the wetting and drying curves of a substrate.
The first step is trying to find a way to measure hydrostatic pressure of a soil if you can't see how high water is drawn up a black soil.
My solution was to dilute 4 yellow highlighter ink's in water, and to use a UV pen light to make the soils fluoresce to see how high they were drawn up black lava rock of various grades. I took course 3 cm grade lava rock and put it through a Blake jaw crusher set to a roughly 2-3 mm setting. The Blake crusher breaks rock into a grade that can pass through it's 2-3 mm aperture. I sifted both crushed lava rock and crushed granite into 2 mm or greater (i.e. gravel), 1- 2 mm, 0.5 - 1 mm, and 0.3 - 0.5 mm sands. I tested these pure mixes and combinations to find out the hydrostatic pressure in a 3x3x18 cm soil column.
On the below left a course soil, mostly gravel (>2 mm), on the right a finer soil with sand ( 0.05 - 2 mm). Water is effectively pulled through a soil by it's adhesion to substrate and it's held back by cohesion to itself.
If you drop a bead of water on a surface, it can stay as a bead as long as the water surface tension, i.e. cohesion, is greater than the water's adhesion to substrate. If you drop a bead of water on a wood table it stays a little dew drop because of cohesion. If you drop a bead of water on cotton fabric, the water adheres to the fine network of fibres and spreads out.
In the illustration below we see capilliary action as a balance between adhesion and cohesion. Gravel has bigger pores, bigger spaces between the gravel particles which allows a balance of cohesion to adhesion such that there is only a small capillary action drawing water up. In sand there is so much negatively charged surface area the water clings to more particles through adhesion and overcomes its surface tension, i.e. cohesion, getting drawn up into the pot higher.
What does this mean in developing a soil to grow lithops? Well in my case I thought it best to test out different grades of porous and non porous substrate to understand the wetting and drying curves of a substrate.
 |
| Black (UV) light pen |
 |
| Fluorescence under UV pen light. |
My solution was to dilute 4 yellow highlighter ink's in water, and to use a UV pen light to make the soils fluoresce to see how high they were drawn up black lava rock of various grades. I took course 3 cm grade lava rock and put it through a Blake jaw crusher set to a roughly 2-3 mm setting. The Blake crusher breaks rock into a grade that can pass through it's 2-3 mm aperture. I sifted both crushed lava rock and crushed granite into 2 mm or greater (i.e. gravel), 1- 2 mm, 0.5 - 1 mm, and 0.3 - 0.5 mm sands. I tested these pure mixes and combinations to find out the hydrostatic pressure in a 3x3x18 cm soil column.
 |
| Equation 1: Forces involved in pore water pressure determination. |
In the above equation we can see that gravity acts on the fluid, we control for the fluid being water by using the density. The units of pore water / hydrostatic pressure are in cm, using cm for height we can see that with a constant density of water, and a constant gravity height is the reactive variable which determines pore water pressure.
Table 1: Experimental Hydrostatic pressure
A fairly imprecise method, but I was surprised at the results, first of all without research I thought the water would not be drawn very high and limited my soil height to ~16 cm, 2cm under water. Several times fluorescence was seen at the soil surface indicating the recorded value is smaller than the correct value if water was allowed to rise through more substrate. Large grade granite was drawing water to the surface of the soil column, as was fine grade and a mix of fine grade granite and basalt.
Granite is composed of hydrous aluminum phyllosilicates, that degrade into negatively charged colloids, and when these fine particles are massed together we know it as clay. These particles are negatively charged and this charge profile may lead to increased forces of adherence.
On the other hand, porous basalt had very low hydrostatic pressures. This may be due to the balance of adhesion to cohesion being lower in the column due to the very high surface area of the porous medium.
To summarise soils exert a pressure on water by adhesion and by capillary action. If the soil has a high hydrostatic pressure -- like peat bogs -- water is drawn closer to the soil surface. If a soil has a low hydrostatic pressure -- like sand dunes -- the soil can only draw water slightly upwards to the surface from the water table. We understand this draw and push as "hysteresis".
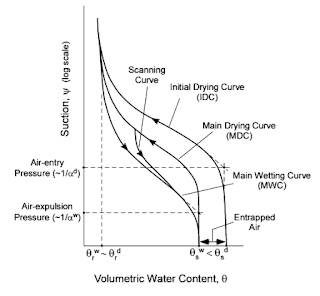 |
Hysteresis and Uncertainty in Soil Water-Retention Curve Parameters, William J. Likos (2014) |
Soils can become fully saturated if the hydrostatic ( suction ) pressure -- measured in cm water above water table -- is strong enough that air is pushed out by higher pressure water. When soil is drying water exerts enough pressure that there has to be suction into the soil to allow air entry. As a soil begins to wet, starting at the top left of the graph above, the pressure pushing it to wet becomes less and less as it reaches its maximum wetness, i.e. high volumetric water content. At one key point air bubbles enter the equation and exert a pressure on the rising water. The water must have a high enough adhesion pressure sucking it upwards to expel air. Conversely as a soil drys there is a key point where air is drawn into a soil overcoming adhesion of the water to the substrate and cohesion to itself.
As in the graph above the air-expulsion pressure is separate from air entry pressure resulting in unique wetting and drying curves of substrates/soils.
Flip the graph above and you get this graph showing how gravel and sand should react.
 |
| A study of infiltration on three sand capillary barriers Hong Yang, H. Rahardjo, E.C. Leong, and D.G. Fredlund. Can. Geotech. J. 41: 629–643 (2004) |
2. Stratification
Many garden centres suggest putting rocks at the bottom of your pot. Why? If you're wicking water from the bottom of the pot, then the moisture level at the top of the course material is as high as the fine material can get. See this fine sand over medium grade sand: |
| A study of infiltration on three sand capillary barriers Hong Yang, H. Rahardjo, E.C. Leong, and D.G. Fredlund. Can. Geotech. J. 41: 629–643 (2004) |
What happens if you do what most people do and water from the top?
Well as you can see, the waters adhesion to the fine material is overcome by gravity and water accumulates in the fine material, more and more as the sum of draining substrate increases, until it reaches gravel where the water is quickly drained by gravity.
So if you wick water upwards you can control the maximum moisture that reaches your plants, the same with watering from the soil surface except you can expect higher volumetric water content in the surface soil than with wicking.
3. Nutrients and pH
Soils are a store of nutrients, but how does it work, and what does pH have to do with it?- Hyaline cells - Peat moss has cells that can store 20x their weight in water, which adds a water sink in the soil
- Organic soils have many organic carbon moieties that can adsorb cations and anions and resist decomposition. This can sink heavy metals and provide nutrients storage and release, as well as buffer pH.
- Organic is known to provide structure and cohesion to loose soil, and act as a binding agent between mineral particles.
Summary:
Wicking is probably best for desert plants as it prevents too much moisture mid-pot in stratified soils as in gravity watering. By wicking we ensure appropriate soil aeration for plant health and fungal resistance.
pH controls which nutrients can be adsorbed to the soil substrate. By having a mix of soil substrates we can allow both anion and cation exchange sites at one pH.
Organic material can bind mineral particles together this gives more stability for roots, and sites for roots to draw nutrients from, but organics can store much more water than mineral soils, making fine root-level moisture control more challenging.
At this point I have some ideas about an ideal soil for Lithops which I'll be explaining in my next blog post about Lithops Germination.
Lithops aucampiae v aucampiae "Storm's Snowcap" C592A © Atreyu 2018
3 Month Seedling Cotyledons in 25% Compost (Bark, Chicken Waste, Municipal Compost) 75% Washed Sand













